Our research is focused on tackling issues such as carbon dioxide reduction, improving grid flexibility, characterizing multiphase flow in reactors, and developing sustainable clothing. We welcome you to take a look at the specific projects below.
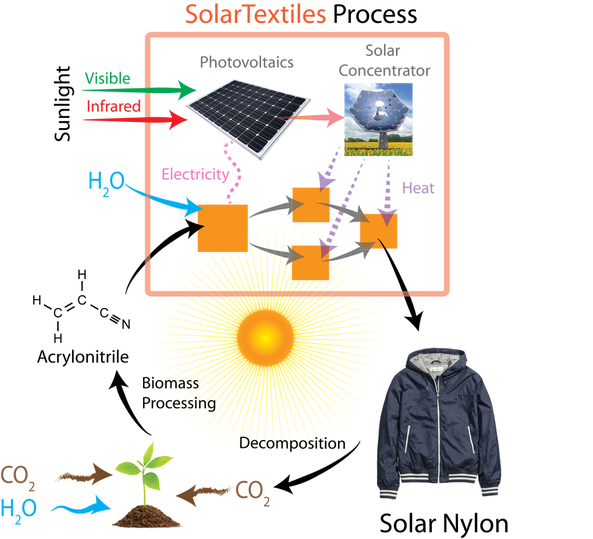
Solar Textiles
The Solar Textiles project explores approaches for the production of polymers from renewable feedstock and solar energy as the only energy input and in this way contributing towards the development of a sustainable chemical industry. The synthesis of Nylon 6,6 has been selected as a model polymer production process, given the importance of the polymer for a wide range of commercial applications, the large scale of the Nylon market (>6 million tons, >USD 20 billion market) and its high value (3.6-4 USD/kg). This makes Nylon a perfect target for large-scale chemical processes that can be adapted to efficiently operate using renewable feedstocks and energy resources. The proposed approach is focused on the combined solar thermo- and electro-chemical synthesis of hexanediamine (HDA), one of the precursors for Nylon 6,6 synthesis. The process is divided by the spectrally selective absorption of UV and Visible irradiation by photovoltaic (PV) arrays, which drive the electrochemical reduction of aqueous streams of acrylonitrile (ACN) to adiponitrile (ADN) and hydrogen (H2). ACN could be sourced from biomass derivatives and be considered a renewable feedstock. The products of the electrochemical reactors will then be fed to a solar thermochemical reactor, which will use the transmitted infrared (IR) radiation from the PV array in a concentrated fashion to carry out the hydrogenation of ADN to HDA in the gas phase at high temperatures (> 300 ˚C). The reactor design principles and integrated solar chemical synthesis processes developed within this proposal, will become the foundation for the conception of a full production process for Nylon 6,6 only requiring the sun, water and CO2 as input. This is a collaborative between the Laboratory of Renewable Energy Science and Engineering at EPFL, and our group at NYU.
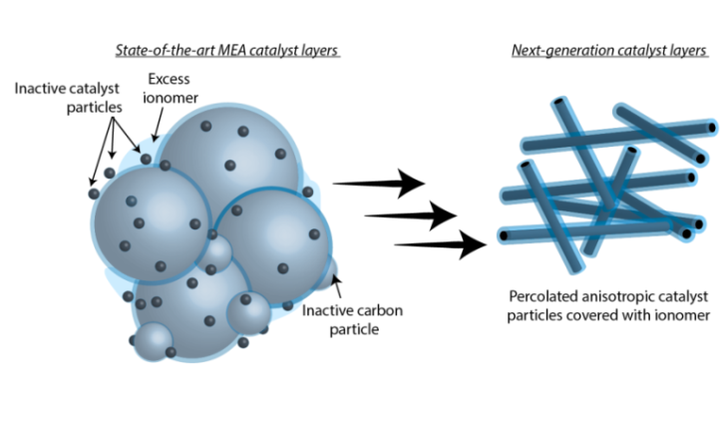
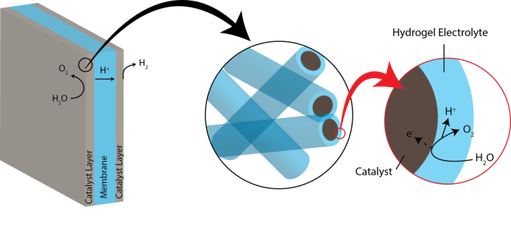
Materials for Electrochemical Catalyst Layers
A major focus of our research is the design of material systems that facilitate pathways for electrochemical reactions. This requires substantial effort towards designing and optimizing catalyst layers, where electrochemical transformations take place. Designing efficient reaction pathways requires understanding the transport requirements of a particular system and developing design rules for the materials in which these processes occur. The bulk of our work investigates liquid and solid electrolytes to emphasize charge and mass transport for water splitting and CO2 reduction applications. With the same concerns regarding the interplay between material, structure, and function, we are investigating methods to precisely and systematically vary the geometry of nanostructured electrodes to gain a more complete understanding of how the nanoscale structure of a catalyst impacts the reaction system.
Advanced Electrolysis Devices
Renewable energy technology has seen massive growth over the last decade due to lower costs and a strong initiative to implement them. However, renewable energy sources such as solar and wind are plagued by intermittency which causes problems when adding these energy sources to the grid. In order to use this energy efficiently, large-scale energy storage systems need to be developed. Our work in this area revolves around the development of new strategies and device designs for efficient and economical energy storage. Redox Flow Batteries (RFBs) are used as the fundamental design component, but we have modified how this electrochemistry is applied. A lot of the research deals with optimizing the operating parameters of the energy storage device and determining, at a more fundamental level, how to improve the efficiency of our systems. Overall, the goal of this research is to develop energy storage systems that could be commercialized for grid-scale applications.
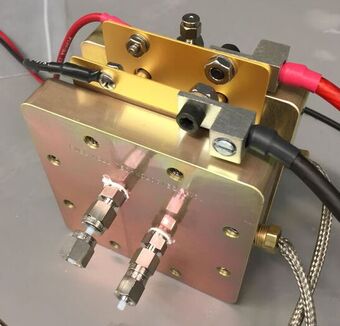
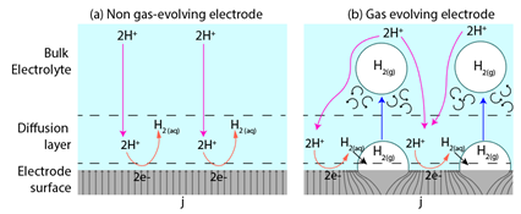
Multiphase-Flow Micro-Electrochemical Reactors
The implementation of Multiphase Flow Electrochemical Reactors (MFER) are an attractive option for efficient and clean hydrogen production by means of water electrolysis. This type of reactors poses an advantage over traditional electrolyzers that use ion conductive membranes that introduce additional resistance to the system, usually work under extreme pH conditions and have limited life cycles, after which they need to be replaced. MFERs are membrane-less, and by understanding of the dynamics of the flowing electrolyte, which allows control over the physical location of gaseous species by fluidic forces without the need for separation membranes, it is possible to achieve high collection efficiencies.
Our group studies the electrochemical operation parameters affect bubble-induced potential losses in water electrolysis in MFERs. An understanding of such impact will allow to develop strategies to manage the potential losses and contribute to the optimization of membrane-less water electrolysis reactors. We believe that by developing the foundations and demonstrating the capabilities of a MFERs, this effort has the potential to impact a broad range of electrochemical processes, which account for more than 5% of the electricity consumption in the world.
Our group studies the electrochemical operation parameters affect bubble-induced potential losses in water electrolysis in MFERs. An understanding of such impact will allow to develop strategies to manage the potential losses and contribute to the optimization of membrane-less water electrolysis reactors. We believe that by developing the foundations and demonstrating the capabilities of a MFERs, this effort has the potential to impact a broad range of electrochemical processes, which account for more than 5% of the electricity consumption in the world.